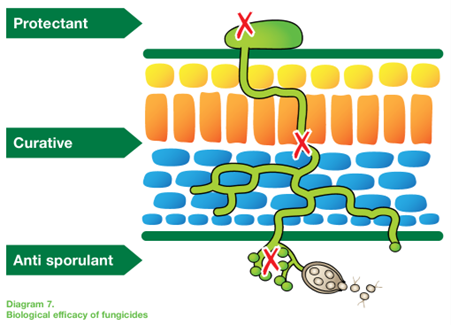
The life cycle
The late blight pathogen can reproduce sexually and asexually. Sexual reproduction allows both parents to contribute genes which results in a new strain with characteristics that differentiate it from both its parents. Asexual reproduction requires only one parent meaning all offspring are clones.
Potato late blight populations are mainly clonal in Great Britain, but sexual reproduction of the pathogen leads to the emergence of novel (non-clonal) genotypes that may have traits such as new virulence or fungicide insensitivity that can make them more challenging to control.
Differences in genotypic diversity have been found between populations of the pathogen in the northern and southern regions of Scotland, with populations in the north-east of Scotland showing greater genotypic diversity.
The frequency of outbreaks defined as non-clonal is circumstantial evidence that sexual recombination of the pathogen has taken place in this region. These feature a high genetic diversity – where up to 50% of samples tested are novel multilocus genotypes – with a unique combination of alleles across all loci.
The north-east of Scotland is primarily a seed potato-producing region, therefore importation of seed from other countries (that may contain novel genotypes) would not exist.
The high levels of non-clonal outbreaks observed may be the result of climatological factors that favour oospore formation, survival and germination. Constant humidity and temperatures of 10–15°C are favourable for oospore germination. Sexual soilborne oospores are likely to be acting as primary inoculum, generating high genotypic variation of the pathogen in this region. It is important to understand the mechanism involved and its potential implications.
Foliar and stem infection via the asexual cycle
The asexual life cycle, (see diagram) begins with sporangia landing on a crop. Under warmer conditions, 18˚C or more, most sporangia germinate directly (1) forming a germ tube that penetrates the epidermal cells. Under cooler conditions most sporangia differentiate to form 10 to 12 motile zoospores that are released through the apex of the spore (2). They move in the water film on the plant surface and are attracted to suitable infection sites where they encyst (3), germinate and form germ tubes that penetrate the epidermis (4).
Unchecked, the pathogen will colonise the plant tissue, form visible lesions and begin to sporulate. Under humid conditions the pathogen forms spore-producing mycelium called sporangiophores that exit the plant.
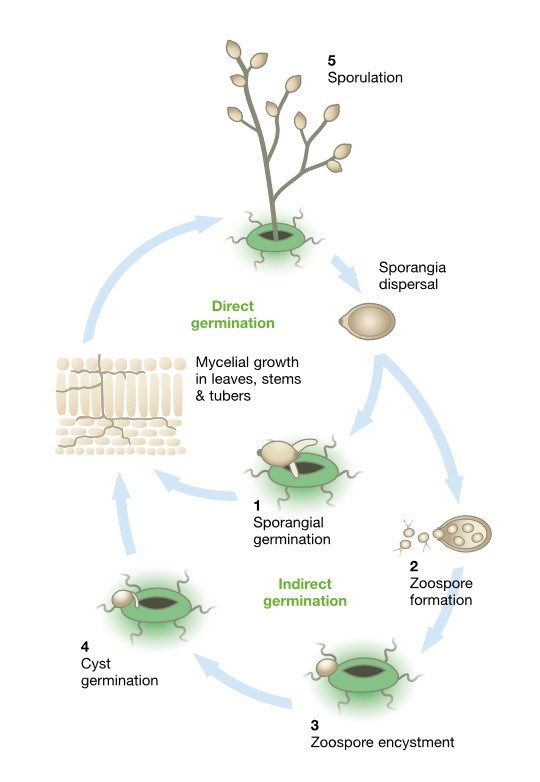
[Illustration 1] The asexual life cycle of potato life blight.
This mycelium appears as a white downy growth on the margins of stem or foliar lesions and generates up to 80,000 sporangia per square centimetre of lesion or 4 x 1012 sporangia per Ha in a single day. Foliar lesions can rapidly defoliate a potato crop, but stem lesions are also particularly damaging, as they are less susceptible to fungicides, can survive dry periods that can check leaf infections and, if they girdle the stem, will kill all the plant tissue above the lesion.
As the life cycle repeats, a focus of blight develops. From this, air-borne spores establish new infections and secondary foci are created. Spores may travel several kilometres and, provided they are not desiccated, or killed by ultraviolet exposure en route, remain viable and can cause infection in other crops.
The length of time from a spore landing on the plant until sporulation begins is termed the latent period. Under optimal conditions the latent period may be as short as three days and if left unchecked explains the explosive epidemic development.

Foliar lesion © Eric Anderson, Scottish Agronomy

Stem blight © Eric Anderson, Scottish Agronomy
Tuber infection
As tubers form, they become vulnerable to infection from sporangia and zoospores. The infection route involves these spores being washed down through the soil, or down the stems themselves (see diagram), or coming into contact with tubers at lifting or grading.
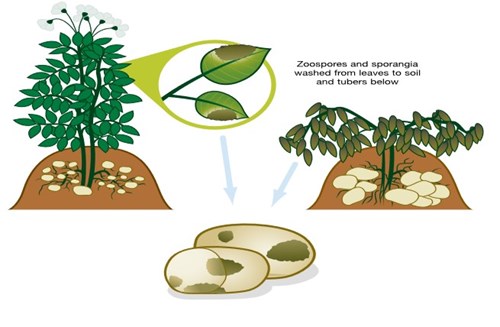
[Illustration 2] Tuber infection
Survival of sporangia in the soil depends on soil type, moisture content and, to a lesser degree, soil temperature. Under natural environmental conditions most sporangia die within 14 days, but they have been found to survive underground for up to 21 days in the absence of green plant material.
Zoospores emerging from sporangia infect tubers and colonise the layer just under the skin. Most infection prior to harvest is through lenticels and eyes leading to tubers with firm chestnut or granular brown lesions just under the skin that spread inwards.
Frequently tuber blight provides an entry route for secondary infection by soft rotting bacteria that convert the flesh to a putrid semi-liquid state.
The main risk factors for tuber infection in the growing crop are the presence of foliar blight, together with high-risk weather to encourage sporulation, followed by lower temperatures to favour the production of a large number of zoospores, combined with wet soil and irrigation or substantial rainfall. Note that for tuber infection, foliar blight does not need to be severe if the other factors are favourable.
Spray intervals of less than seven days will be required where active blight is present until control is regained. This is likely to require alternating products to ensure adherence to the spray intervals specified on the product label. Ensure that haulm coverage is optimised by selecting appropriate nozzles and using a higher volume of water than normal. To minimise the selection for insensitive strains, alternate products with different modes of action (FRAC codes) and/or use tank mixtures of active substances, or co- formulations, with different FRAC codes.
Where possible avoid irrigating shortly after periods of high-risk weather, i.e. warm and humid conditions. The combination of weather conducive to the production of sporangia followed shortly after by irrigation substantially increases the risk of tuber infection because spores are washed down into the soil. The decision over whether to irrigate or not can be easier to make if there is good information on the extent of foliar blight in the crop. The implications for tuber infection are clearly different if foliar blight is restricted to one or two small, localised patches compared with it being present throughout the field.
Minimising the passage of blighted tubers into store
Remember that the role of fungicide applications is not just to protect tubers; it is also to reduce the amount of viable inoculum in the crop. Consolidating cracked ridges with a ridge roller after initial haulm desiccation and ensuring complete haulm desiccation to prevent re-growth can reduce the risk of tuber blight.
If test digs ahead of haulm destruction reveal blighted tubers, then the number making it into store can be reduced by delaying harvest and allowing them to rot induced by secondary bacterial infection. However, it should be noted that the success of this approach is greater for soils that are warmer and wetter. Experience suggests that on occasions tuber decay can be limited in free-draining soils, especially once soil temperatures fall significantly.
Under warm and humid or wet soil conditions, a large number of spores can be produced on regrowth, which subsequently infect tubers damaged during harvest. To minimise the risk of this, check that desiccation was as effective as expected and ensure that haulm has been completely dead for at least 14 days.
Where tuber blight is visible it would be prudent to leave crops for at least 21 days. Although the number of viable spores in the soil decreases quickly after desiccation it is many weeks before the number declines to zero. If blighted tubers get into store and remain moist, this greatly increases the risk of secondary bacterial soft rots. If a crop with tuber blight is harvested before a healthy crop, ensure that the harvester is thoroughly cleaned between crops.
ASSESSING TUBER BLIGHT LEVELS BEFORE HARVEST
- Use hot box with temperature set at 20°C for 12-18 hours.
- Random sample of 300 tubers required to detect 1% infection with confidence.
- Completely peel and cut all tubers to assess potential infection.
Prevention of tuber blight during harvest
- Ensure thorough haulm destruction, and check crops for haulm regrowth, to prevent re- growth that is blighted spreading blight spores onto tubers on the harvester.
- For crops that have, or have had, foliar blight, if the variety is particularly susceptible to tuber blight, then consider lengthening the period between desiccation and harvest for an extra week or two to allow a greater number of viable spores in the soil to die off. Fewer viable spores are required to infect tubers of tuber-susceptible cultivars.
- Don’t let harvested tubers become wet.
- Ventilate and dry tubers immediately after harvest to avoid condensation on tuber surfaces.
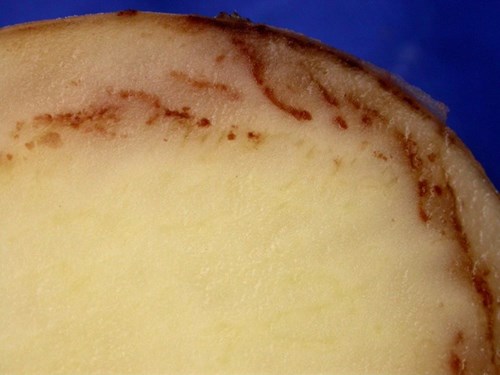
Early symptoms of tuber blight© Eric Anderson, Scottish Agronomy
The following factors increase the risk of tuber blight and a combination of several of them is required for tuber blight to occur:
- Haulm with foliar blight where risk increases with rising levels of inoculum.
- High risk weather that maximises spore production on haulm.
- A temperature drop below 11°C that encourages formation of zoospores which are smaller, motile in water and therefore more likely to reach tubers.
- Rainfall or irrigation of 5 mm or more that washes spores into soil.
- High soil moisture content which favours tuber infection – lesion development increases from 40 to 80% soil field capacity.
- Cracked ridges and shallow-set tubers effectively shortening spores’ journey from leaf to tuber.
- Sandy soils which zoospores can travel faster through than clay soils.
The asexual form of Phytophthora infestans will not survive between seasons in the absence of live potato tissue, so fully rotted tubers break the cycle. Conversely, infected tubers that survive can carry primary inoculum to re-start the cycle the following season.
Oospores and the sexual life cycle
The phases of the life cycle described above are the result of asexual (i.e. clonal) reproduction. Oospores can be an advantage to the pathogen as successful combinations of traits remain genetically fixed for months or even years. However, this limits the opportunities for genetic evolution which is a disadvantage over the longer term.
The pathogen has two mating types termed A1 and A2 and, as illustrated in the diagram, these opposing forms must co-infect and meet in plant tissue (6) to reproduce sexually. Until the 1970s this part of the life cycle could not occur in Europe as the A2 type was only found in Mexico, the pathogen’s centre of origin. With the introduction and spread of the A2 mating type in Europe, both types may now co-infect plants and reproduce sexually, generating thick-walled oospores (7). These oospores enter the soil as the infected plant material decomposes, remain viable for many years and then germinate (8) in the presence of a host plant to form sporangia (9) which re-start the life cycle. Oospores can survive in a field under very different climatic conditions from several months up to four years. Indications of sexual reproduction, resulting in soil-borne inoculum were first reported from Sweden. There are also observations of primary infections probably arising from oospores in Denmark and Finland.
The diagram combines the asexual and sexual life cycles of the late blight pathogen and shows how they drive disease development in leaves, stems and tubers.
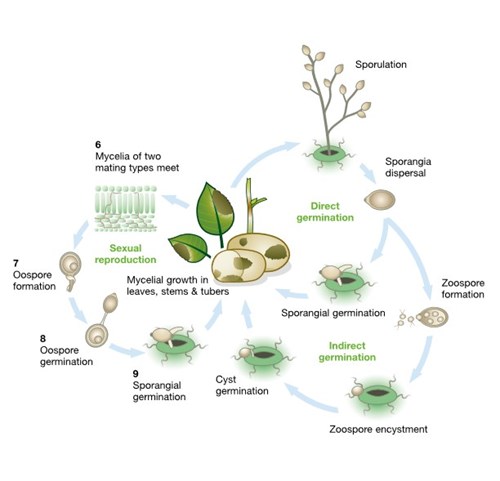
[ILLUSTRATION 3] The full life cycle of potato late blight
Each germinating oospore is genetically distinct, and such ‘re-shuffling of the genetic pack’ generates new combinations of traits. Through a process of natural selection those genotypes that are more aggressive, fitter, resistant to fungicide or more capable of overcoming host resistance than the existing pathogen population, will be more likely to spread and cause crop disease that is more difficult to manage.
The implications of oospores acting as a source of inoculum are potentially serious. In other parts of Europe, such as in the Nordics and The Netherlands, early infections from soil-borne oospores have proved difficult to manage.
In British crops, however, despite the prevalence of the A1 and A2 mating types, oospores have not yet been found. It is almost certain that they are there, but we just haven’t looked for them. There is an increasing body of evidence to show that oospores could be an important source of primary infection. In the North of Scotland, the presence of “Other” or “Miscellaneous” genotypes in Fight Against Blight reporting is evidence enough of sexual recombination occurring.
An important factor is the length of the rotation between potato crops. In the absence of a susceptible crop the viability of dormant oospores declines, and the reduced inoculum load thus decreases disease risk. Maintaining long rotations is therefore advisable for managing late blight as well as other soil-borne pests and pathogens.
Nonetheless, growers should remain alert to the signs of soil-borne oospore inoculum, particularly in crops grown in fields that suffered severe blight infection when potatoes were last grown. Warm, wet conditions after planting stimulate co-ordinated oospore germination that can cause patches of sudden infection of the lower leaf canopy of the emerging crop.
Signs to look for include the early appearance of multiple blight lesions on leaves in contact with the soil and patches of young plants dying-off from stem blight that appears to start from the stem base or below the soil. This may occur in low-lying areas of the field where wet soil conditions favour pathogen activity.